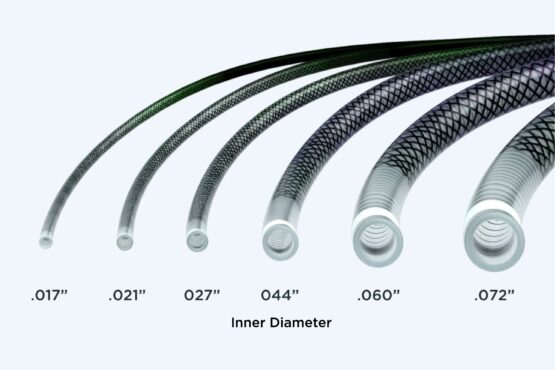
eNVoke™ Catheter
CE marked product information
Intended Use
The eNVoke™ Catheters are intended for the introduction of interventional devices and infusion of diagnostic or therapeutic agents into the neuro, peripheral, and coronary vasculatures.
Contraindications
None known
Warnings
- Do not use if damage to the device is observed.
- Do not use if the product sterile barrier system or its packaging is compromised.
- This device is intended for single use only. Do not resterilize and/or reuse in multiple patients. Structural integrity, sterility and/or function may be impaired by resterilization or re-use.
- Never advance or withdraw an intravascular device against resistance until the cause of resistance is determined by fluoroscopy. Movement of the device against resistance could dislodge a clot, perforate a vessel wall, or damage the device.
- The catheter should be manipulated under fluoroscopy only. Do not attempt to move the catheter without observing the resultant tip response.
- Do not exceed 2070 kPA (300 psi) maximum recommended infusion pressure. Excess pressure may result in catheter damage or patient injury. Use of power injectors requires careful monitoring of catheter tip placement in the vasculature to avoid vessel damage.
- Do not exceed 317 kPa (46 psi) if lumen of catheter is occluded as this may result in catheter damage or patient injury.
- The safety and effectiveness of the device has not been established, or is unknown, in vascular regions other than those specifically indicated.
- This device is coated with a hydrophilc coating at the distal end of the device for a length indicated on the label. Please refer to Procedure section for further information on how to prepare and use this device to ensure it performs as intended. Failure to abide by the warnings in this labeling may result in damage to the device coating, which may necessitate intervention or result in serious adverse events.
Precautions
- Carefully read these directions before using this product. Observe warning and safety precautions.
- The appropriate anti-platelet and anti-coagulation therapy should be administered in accordance with standard medical practice.
- The device should only be used by physicians experienced in angiographic and percutaneous interventional procedures, at medical facilities with the appropriate fluoroscopic equipment.
- Use device prior to “Use-by” date printed on label.
- Prevent exposure to temperatures in excess of 55°C. Exposure to temperatures above this temperature may damage device and accessories. Do not autoclave.
- To prevent thrombus formation and contrast media crystal formation, maintain a constant infusion of appropriate flush solution between guide catheter and microcatheter and between the microcatheter and guidewire.
- If flow through catheter becomes restricted, do not attempt to clear catheter lumen by infusion. Doing so may cause catheter damage or patient injury. Remove and replace catheter.
- Torqueing the catheter may cause damage which could result in kinking to separation of the catheter shaft.
- If an intraluminal device becomes lodged in the catheter, or if the catheter becomes severely kinked, withdraw the entire system (intraluminal device, catheter, and guiding catheter).
- The Shaping Mandrel is not intended for use inside the body. Ensure Shaping Mandrel is removed from the catheter prior to introduction into the RHV or other accessories.
- Use only a steam source to shape the catheter tip. Do not use other heat sources or the catheter may be damaged.
- Verify that the diameter of any guidewire or accessory device that is used is compatible with the inner diameter of the catheter prior to use.
- The eNVoke™ Catheter has a lubricious hydrophilic coating on the outside of the distal end.
- The coating must be kept hydrated in order to be lubricious.
- The coating is incompatible with solvents such as alcohols or cleaning agents. Avoid using alcohols, antiseptic solutions, or other solvents as these may damage the coating, which could affect device safety and performance.
- Avoid wiping the device with dry gauze as this may damage the device coating. Avoid excessive wiping of the coating.
- Avoid device insertion through a metal cannula or needle. Manipulation, advancement, and/or withdrawal through a metal device may result in coating material remaining in the metal device leading to adverse events such as embolization.
- Caution: Federal (USA) law restricts this device to sale, distribution and use by or on the order of a physician.
Potential Complications
Possible complications of the use of the eNVoke™ Catheter include, but are not limited to:
- Adverse reaction to antiplatelet/anticoagulation agents or contrast media
- Additional surgical Intervention
- Allergic reaction
- Aneurysm or pseudoaneurysm
- Aneurysm perforation or rupture
- Anesthesia complications
- Arteriovenous fistula
- Cerebral infarct
- Change in mental status
- Death
- Device deformation, collapse, fracture, or malfunction
- Edema, including braid and pulmonary
- Embolization (air, tissue, or thrombotic emboli)
- Hematoma at the site of entry
- Hemorrhage
- Hypotension/hypertension
- Infection
- Inflammation, including sterile inflammation or granulomas at the access site
- Intracranial hemorrhage including subarachnoid hemorrhage and hemorrhagic transformation
- Ischemia
- Myocardial embolism
- Myocardial infarct
- Neurological deficits including stroke and death
- New stroke/ cerebrovascular accident/transient ischemic attack (TIA)
- Pain at the site of entry
- Post procedural bleeding
- Pulmonary embolism
- Pulmonary infarct
- Pseudoaneurysm
- Renal failure
- Respiratory failure
- Shock
- Stroke
- Thrombosis (acute and subacute)
- Tissue necrosis, transient or long-lasting
- Vascular thrombosis
- Vascular occlusion
- Vasoconstriction (Vasospasm)
- Vessel trauma, dissection, perforation, rupture, or injury